Abstract
In this experiment, we shall investigate the
wave nature of moving electrons, and interference of electron waves diffracted
by polycrystalline layers of graphite. The bright rings produced by interference
of electron waves will be used to determine the d-spacing values characteristic
of the graphite crystal structure.
Introduction
It was hypothesized in a Ph.D. thesis provided
by de Broglie that moving particles are associated with the propagation of
waves. The wave length of a particle moving with momentum p is given by
equ.1. where h is Planck’s constant. The wave nature of moving electrons
was later demonstrated by experimental work of Davisson and Germer.
When parallel rays are incident on a set
of scattering atomic planes in a crystal, they are reflected by these planes.
Constructive interference is then observed in particular directions, forming a
spot on a screen. The angle of scattering in the direction of the spot is
determined by Bragg’s law equ.2 , see Fig.1.
Figure 1: Bragg’s law
Here ɵ is the angle between the incident or reflected
rays and the scattering lanes. The scattering angle (between the incidence and
scattering directions) is 2θ. Usually, the first order reflection (n =
1) is dominant, and higher order reflections are weak. If we have a
polycrystalline sample, with crystal planes oriented randomly in space, a ring
is observed as a consequence of constructive interference of waves scattered
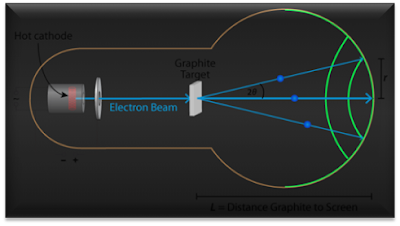
from a given set of planes. Fig. 2 shows the diffraction process in the
electron diffraction tube of radius R, and one of the observed rings of
radius r on the screen.
Figure 2: Diffraction
ring due to constructive interference of electron waves
scattered by a polycrystalline sample.
Graphite Structure
Graphite crystal consists of layers of carbon atoms arranged at the vertices of hexagons, each layer having a honeycomb 2-dimensinal arrangement as in Fig. (3). Atoms in the same layer are boded covalently, while different layers are attracted to each other by van der Waal’s forces. The nearest neighbor distance between two atoms in a layer is 1.42 Å, while the c-axis length (perpendicular to the layers) is 6.88 Å. The first two sets of planes with their respective separations are shown Fig. 3 &4. These separations can be easily calculated from the geometry, and are given by equ.7 and equ.8.
Figure 3 &4: Honeycomb
arrangement of carbon atoms in a graphite layer.
Methodology
Formalism
The wave length of a particle moving with momentum p , where h is Planck’s
constant.
Bragg’s law:
2𝑑𝑠𝑖𝑛𝜃 = 𝑛𝜆 ………………… (2)
𝑟 = 𝑅𝑠𝑖𝑛(4𝜃) ………………. (3)
𝑟 = 4𝑅𝑠𝑖𝑛𝜃 ………………………. (4)
𝑑1 = 1.42(1 + sin(30)) = 2.13 Å ……..(7)
𝑑2 = 1.42 sin(60) = 1.23 Å …………...(8)
Data Analysis
|
V (KV) |
d 1( in) (cm) |
d 1 (out)(cm) |
d 2 (in) (cm) |
d 2 (out)(cm) |
Average
diameter (in) (cm) |
Average
diameter (out) (cm) |
|
4 |
2.05 |
2.66 |
3.75 |
4.53 |
2.355 |
4.140 |
|
4.5 |
1.94 |
2.46 |
3.52 |
4.30 |
2.220 |
3.911 |
|
5 |
1.96 |
2.36 |
3.46 |
4.20 |
2.160 |
3.832 |
|
5.5 |
1.85 |
2.19 |
3.20 |
3.75 |
2.020 |
3.475 |
|
6 |
1.74 |
2.01 |
3.06 |
3.50 |
1.870 |
3.280 |
|
6.5 |
1.70 |
1.99 |
3.00 |
3.35 |
1.845 |
3.175 |
|
7 |
1.62 |
1.90 |
2.90 |
3.20 |
1.760 |
3.050 |
|
7.5 |
1.51 |
1.70 |
2.74 |
3.19 |
1.600 |
3.963 |
|
8 |
1.48 |
1.67 |
2.70 |
3.02 |
1.570 |
3.861 |
|
8.5 |
1.40 |
1.63 |
2.60 |
2.90 |
1.515 |
3.750 |
|
9 |
1.39 |
1.61 |
2.60 |
3.10 |
1.500 |
2.851 |
|
9.5 |
1.30 |
1.53 |
2.57 |
3.10 |
1.410 |
2.834 |
|
10 |
1.28 |
1.57 |
2.50 |
3.00 |
1.420 |
2.752 |
Using the value for the tube radius R = 6.5 cm, the radii of the rings,
and the equation:
We can calculate the range of the angle θ
And by using the equation:
We can calculate the wavelength for each accelerating voltage. The results are in the table in the following page.
|
r 1 |
r 2 |
θ1 |
θ2 |
𝜆(A m) |
|
1.17 |
2.071 |
2.579 |
4.568 |
0.193848 |
|
1.10 |
1.955 |
2.424 |
4.3122 |
0.182761 |
|
1.08 |
1.915 |
2.380 |
4.2238 |
0.173383 |
|
1.01 |
1.737 |
2.226 |
3.830 |
0.165314 |
|
0.93 |
1.642 |
2.049 |
3.620 |
0.158276 |
|
0.92 |
1.587 |
2.027 |
3.499 |
0.152067 |
|
0.88 |
1.525 |
1.939 |
3.3625 |
0.146535 |
|
0.80 |
1.482 |
1.763 |
3.267 |
0.141566 |
|
0.78 |
1.432 |
1.719 |
3.157 |
0.137071 |
|
0.75 |
1.375 |
1.652 |
3.031 |
0.132978 |
|
0.75 |
1.425 |
1.652 |
3.141 |
0.129232 |
|
0.70 |
1.411 |
1.542 |
3.110 |
0.125785 |
|
0.71 |
1.375 |
1.564 |
3.031 |
0.1226 |
For the first ring
|
Radius for the first ring
(cm) |
Wavelength (A) |
|
1.17 |
0.193848 |
|
1.10 |
0.182761 |
|
1.08 |
0.173383 |
|
1.01 |
0.165314 |
|
0.93 |
0.158276 |
|
0.92 |
0.152067 |
|
0.88 |
0.146535 |
|
0.80 |
0.141566 |
|
0.78 |
0.137071 |
|
0.75 |
0.132978 |
|
0.75 |
0.129232 |
|
0.70 |
0.125785 |
|
0.71 |
0.1226 |
The slope of the straight line = 6.9711 *10^8 m
The relation is linear and direct.
For the second ring
|
Radius
for the second ring (cm) |
Wavelength
(A) |
|
2.071 |
0.193848 |
|
1.955 |
0.182761 |
|
1.915 |
0.173383 |
|
1.737 |
0.165314 |
|
1.642 |
0.158276 |
|
1.587 |
0.152067 |
|
1.525 |
0.146535 |
|
1.482 |
0.141566 |
|
1.432 |
0.137071 |
|
1.375 |
0.132978 |
|
1.425 |
0.129232 |
|
1.411 |
0.125785 |
|
1.375 |
0.122600 |
The slope of the straight line = 10.342 *10^8 m
The relation is also linear and direct.
From the slopes in step (3) above, the d- spacing for each ring can be calculated using the equation:
For the first ring, the d-spacing is:
d1 = 1.864A
The real value = 2.14 A
So, the percentage error is:
For the second ring, the d-spacing is:
d2 = 1.211A
The real value = 1.2570 A
So, the percentage error is:
Discussion
We increased the retarding potential and measured the inner and the outer diameters of each ring, then we calculated the radius and the range of the angle θ using the equation: r = R Sin (θ), where R is the radius of the tube. We also calculated the wavelength for each accelerating voltage using the equation:
Then, we plotted the ring radius versus the wavelength for the two rings
and determined the slope of the straight lines.
For the first ring, the slope equals 6.9711 *10^8 m
For the second ring, the slope equals 10.342 *10^8 m
Conclusion
In this experiment we used an evacuated tube which contain an electron
emitter that provides electron beam , it also contains an accelerating
anode to provide a known energy value to
the electrons in the beam , crystal targets made of either aluminum or graphite
; and an observing screen for viewing the electron diffraction pattern.
The relation between the radius of the ring and the wavelength of the
incident light is linear and direct, which means that as the wavelength of the
incident beam increases, the radius of the ring also increases.
The diffraction pattern observed on the screen is a series of concentric
rings. This is due to the regular spacing of the carbon atoms in different
layers in the graphite. However, since the graphite layers overlay each other
in an irregular way the resulting diffraction pattern is circular.
We notice also that when the accelerating voltage is increased, the radius of the rings decreases because the energy of the electron increases.
References
1- ADVANCED PRACTICAL PHYSICS
(0352311), Sami Mahmood, The University of Jordan.





.png)
.png)
.png)


.png)
.png)
.png)
.png)
Comments
Post a Comment
If you have any comment please write it down, we will happy to read it.